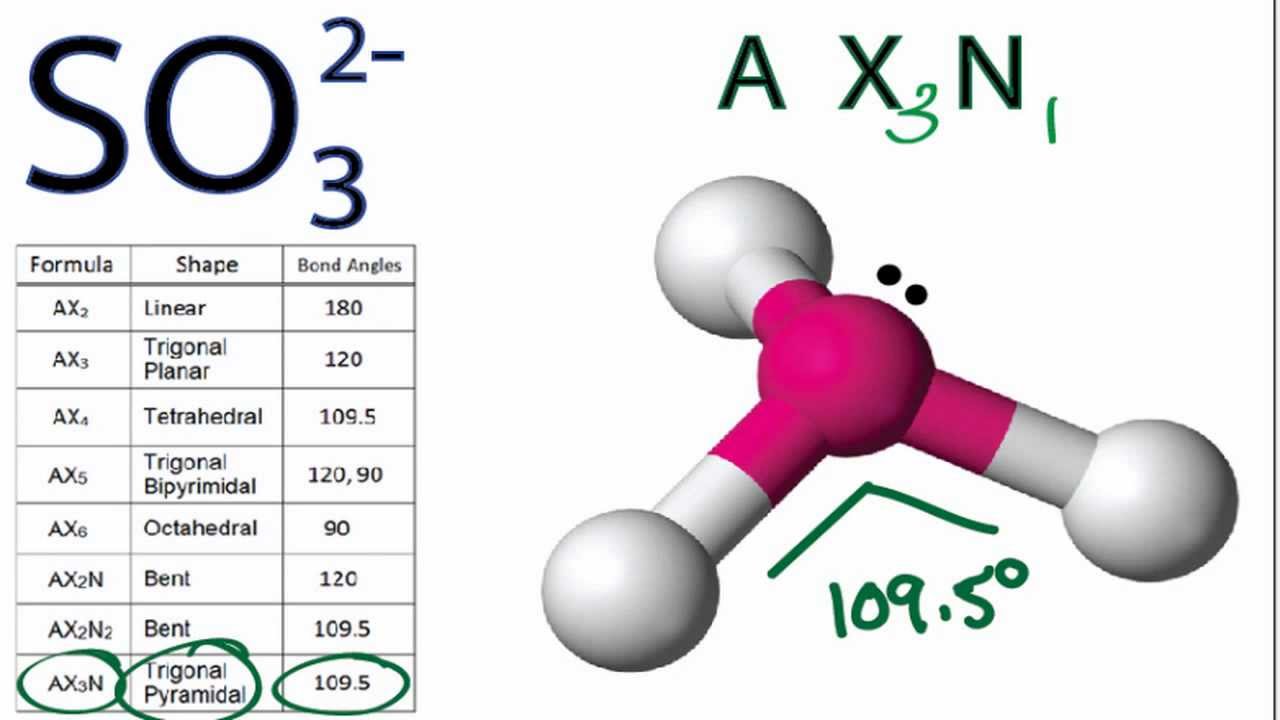What are the shapes and hybridization of the following molecule or ions, (1) SO3, (2) XeF2, (3) SCl2? - Quora

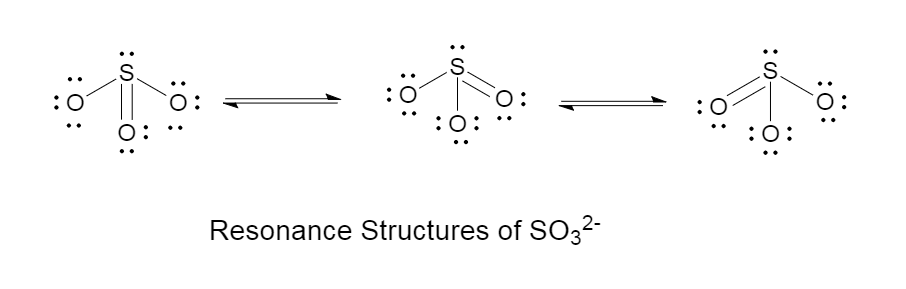
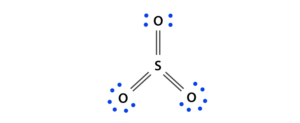
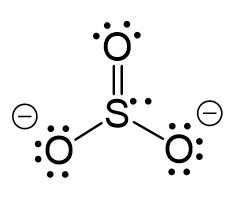
Draw the Lewis structure for SO32-. How many bonds and non-bonding pairs are around the central atom? What is the shape of this molecule? | Homework.Study.com

SO3 Hybridization (Sulfur Trioxide) | SO3 Hybridization (Sulfur Trioxide) Are you searching for a video to help you understand SO3 Hybridization? If yes then check out this video to know our...

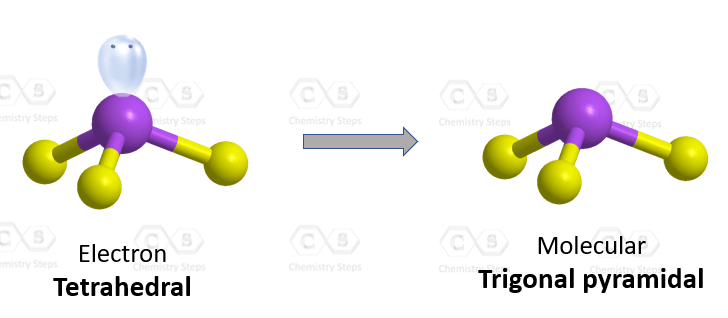
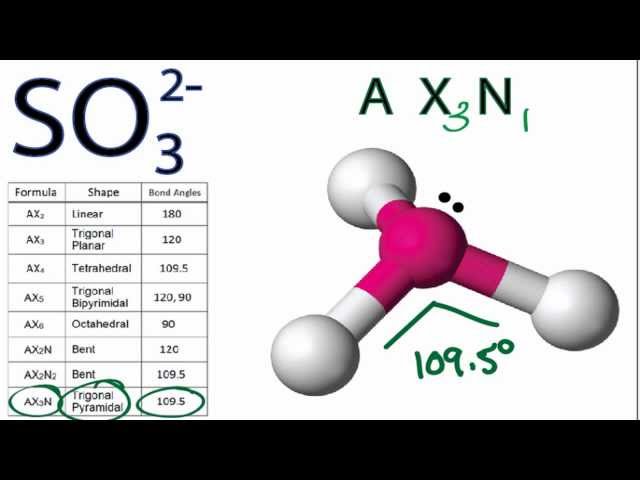
Draw the Lewis structure for SO32- and provide the following information. a. electron geometry b. molecular geometry c. hybridization d. polarity | Homework.Study.com

What is the hybridization of the sulfur atom, electronic geometry, and molecular geometry in SO3^2 ion? | Homework.Study.com
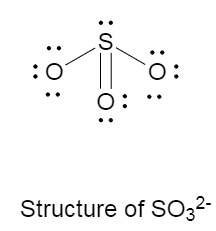
In $SO_3^{2 - }$ :A.$d\\pi - p\\pi $ bond between $S$ and $O$ is delocalized.B.Bonds between $S$ and $O$ are equivalent.C.There is $s{p^3}$ hybridized sulphur atomD.All of the facts given above are

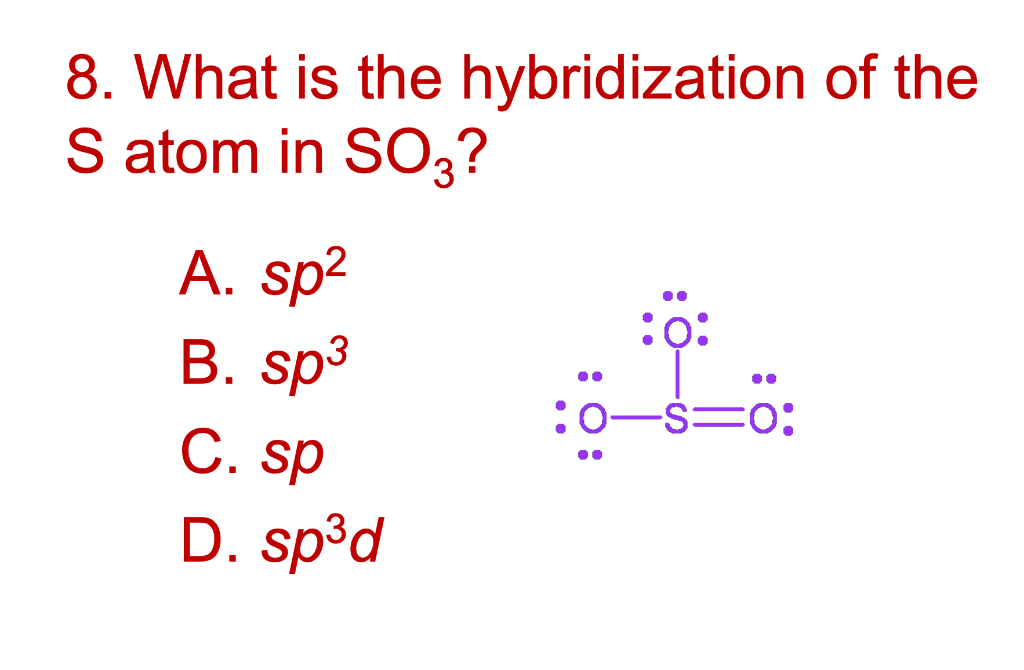
SOLVED: Question 1 (2 points) Consider the Lewis Structure of the sulfite ion, SO32- What would be the hybridization around the central atom in this polyatomic ion? sp Sp2 sp3 sp

SO32- Lewis structure, molecular geometry or shape, bond angle, hybridization, | Molecular geometry, Molecular, Electron configuration