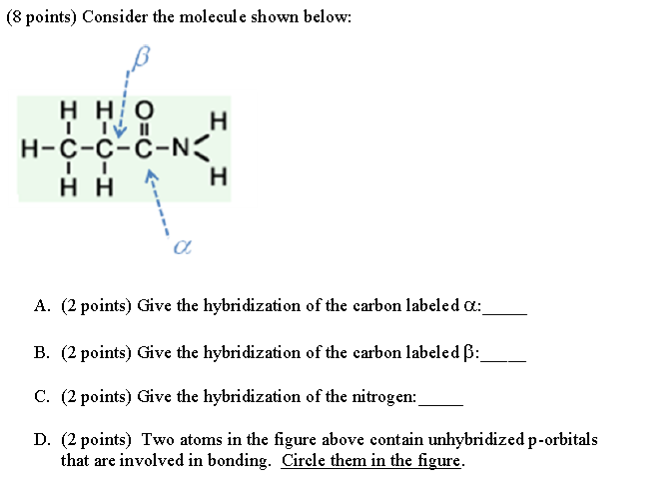
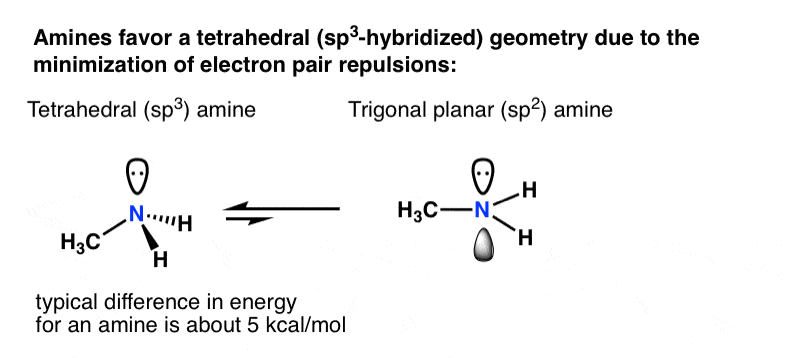
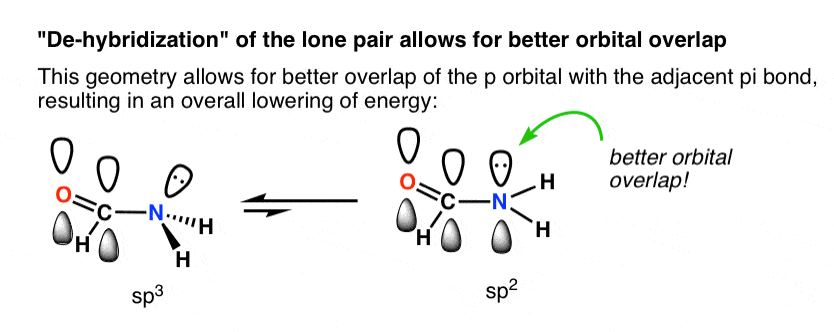
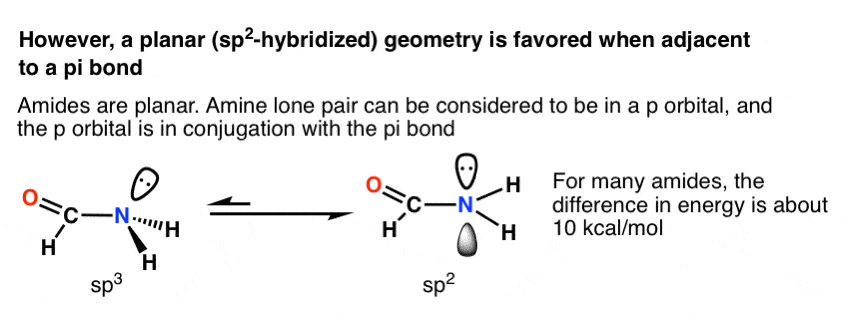
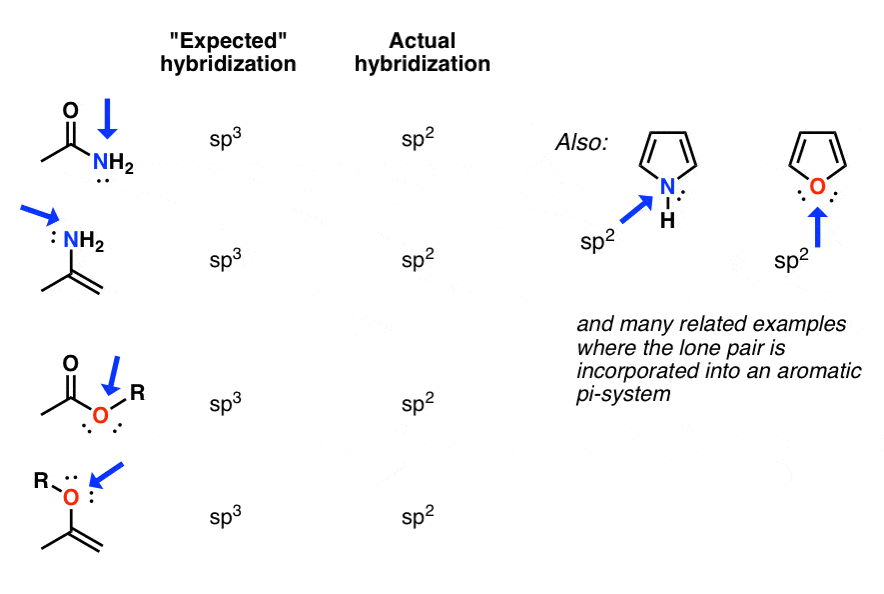
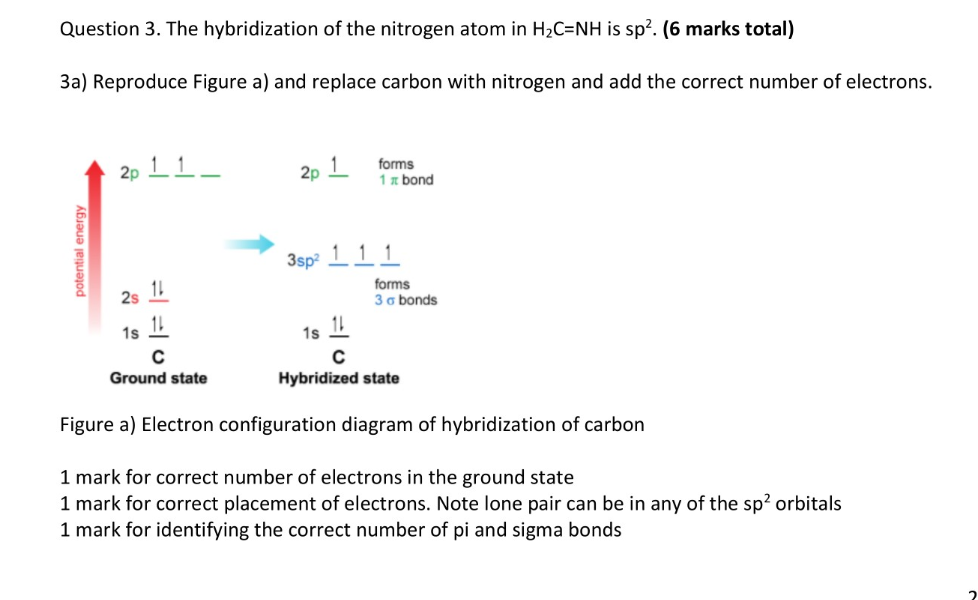
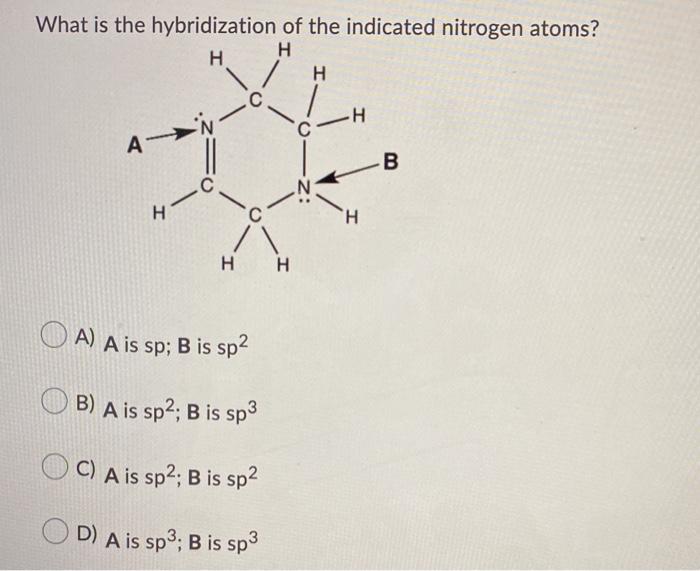
Why is this nitrogen sp2 hybridized? Wouldn't the first structure be more stable bc less formal charges? : r/OrganicChemistry

Environment of the nitrogen atom with sp3 orbital hybridization (cf.... | Download Scientific Diagram

SOLVED: What is the hybridization state of the nitrogen atom in the following compound? H₈-N sp² sp³ sp⠴

The hybridization/s of N in solid { N }_{ 2 }{ O }_{ 5 } is/are:{ sp }^{ 3 } , { sp }^{ 2 }sp , { sp }^{ 2 }sp , { sp }^{ 3 }d{ sp }^{ 3 }

The hybridization states of the nitrogen atom in pyridine piperidine and pyrrole are respectively - YouTube

NO2 Hybridization (Nitrogen Dioxide) | NO2 Hybridization (Nitrogen Dioxide) Nitrogen Dioxide or Nitrogen oxide is a molecule that consists of one Nitrogen and two Oxygen atoms. In this video... | By Geometry