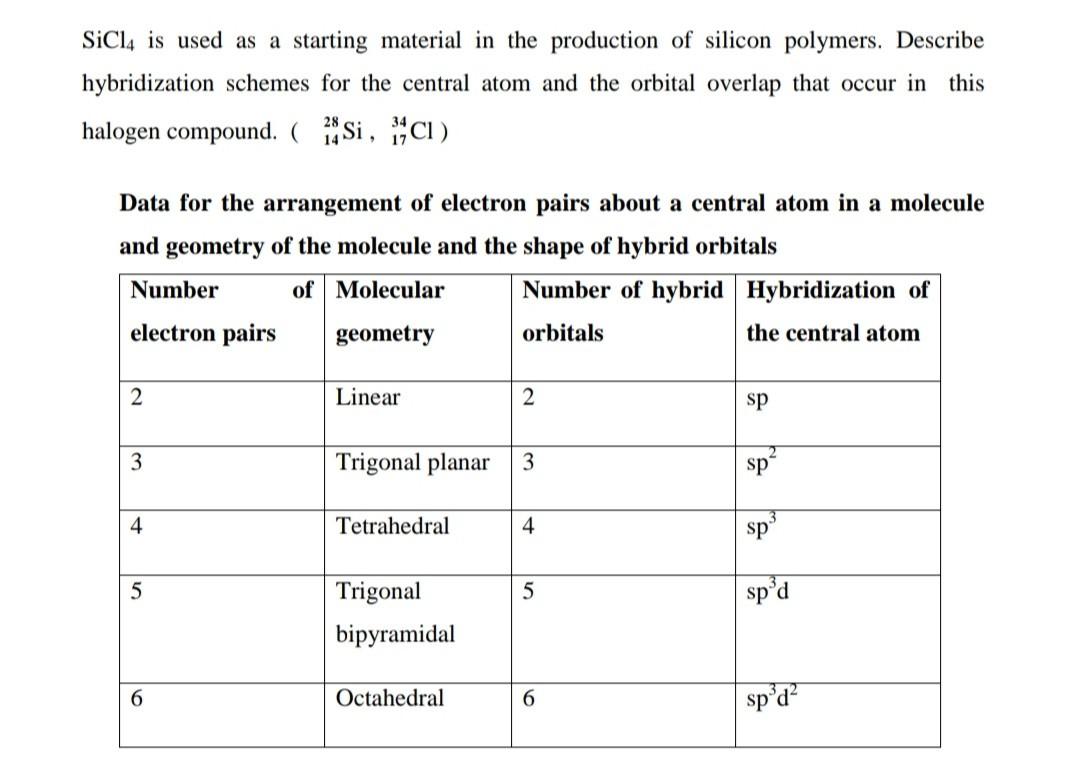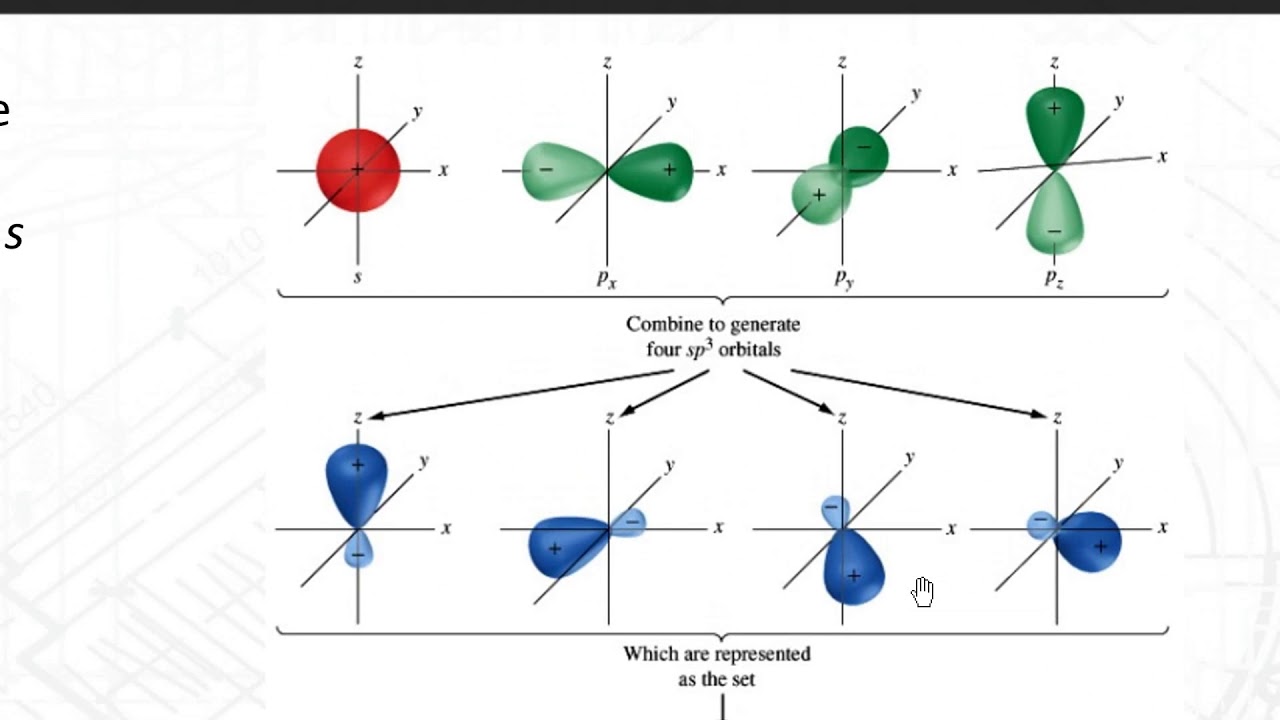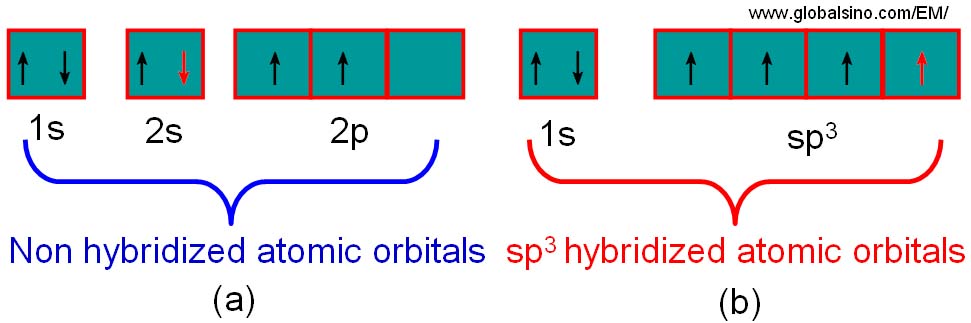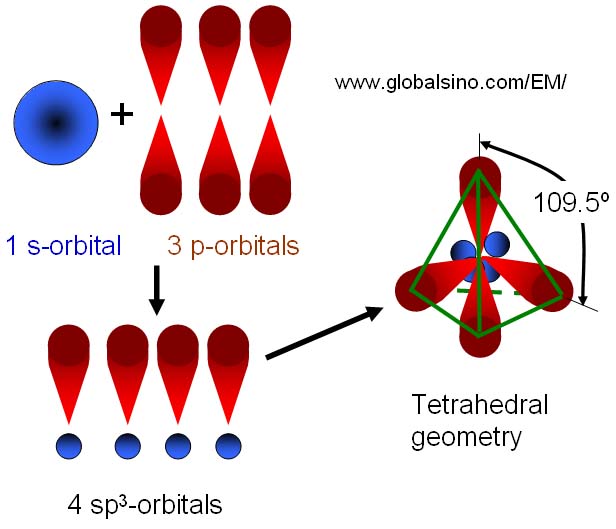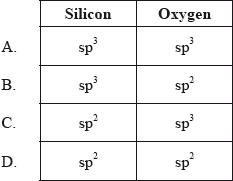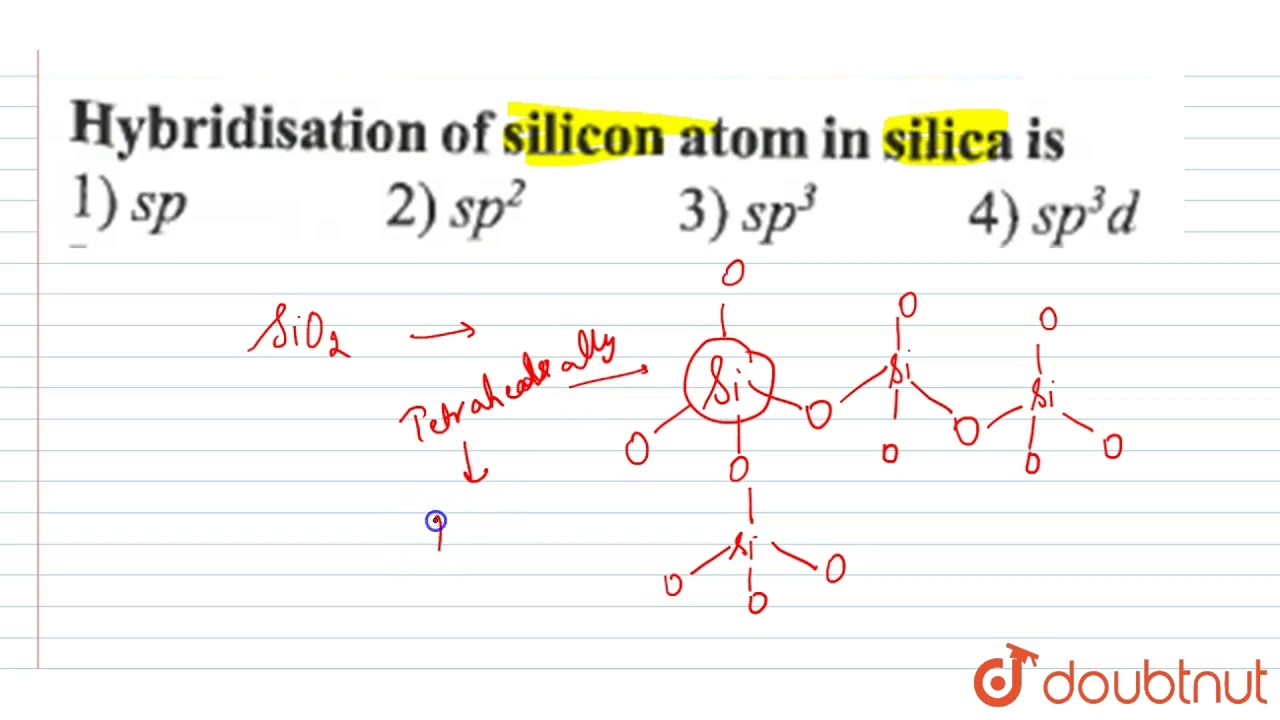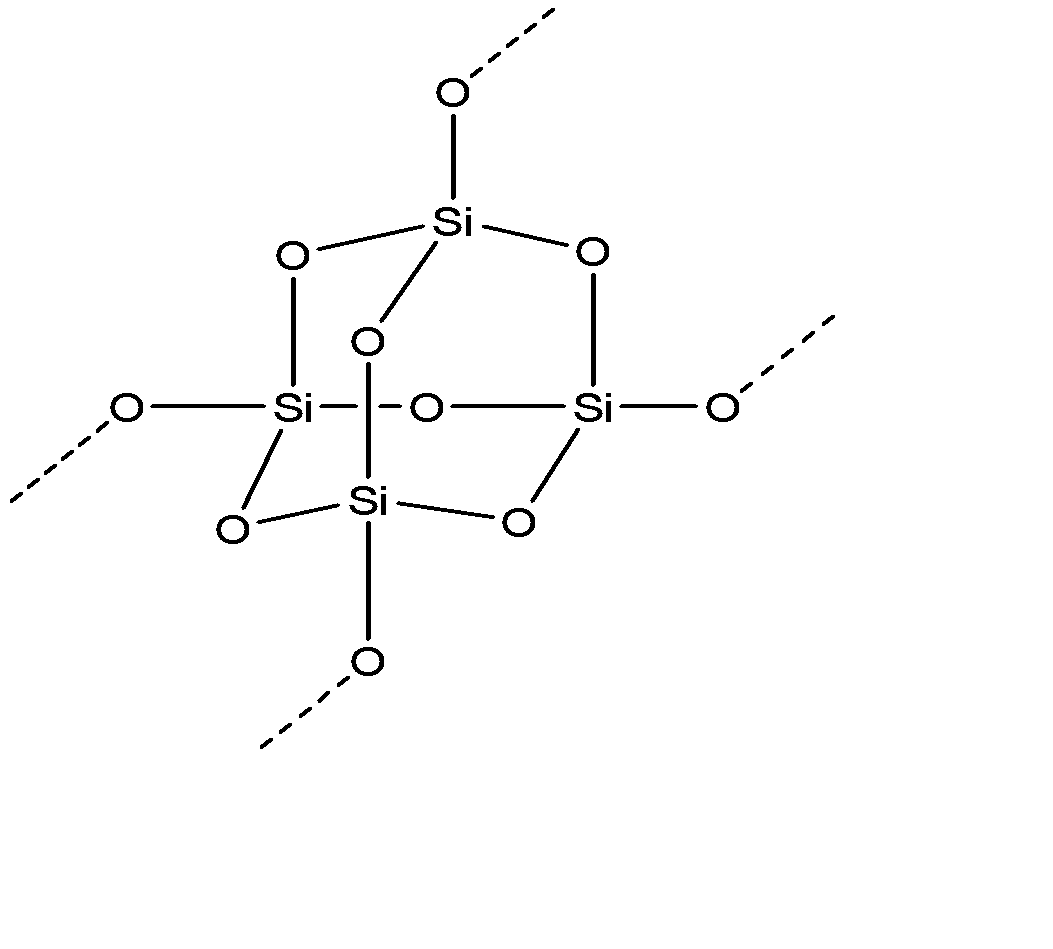
Hybridization of silicon atom in silica is:(A) sp(B) $s{{p}^{2}}$ (C) $s{{p}^{3}}$ (D) $s{{p}^{3}}d$
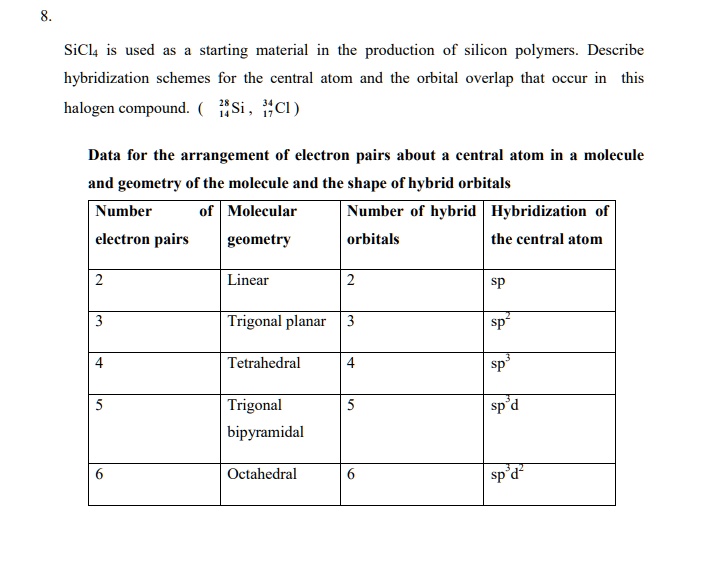
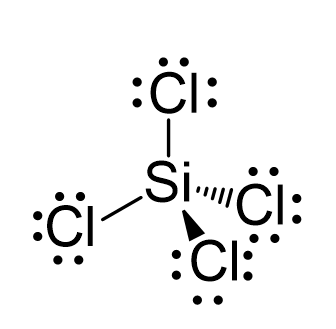
SOLVED: The production of silicon polymers is described. Hybridization schemes for the central atom and the orbital overlap that occur in this halogen compound (SiCl4) are discussed. Data for the arrangement of