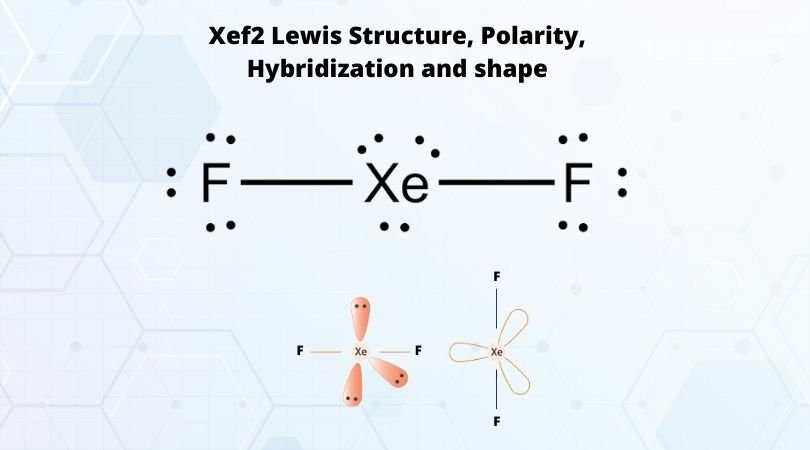
What is the shape and hybridization in XeO3 , ClF5, IF3, XeF2, SF4, PCl5, P2O5, XeF4, XeF6, ICl4 - - Chemistry - The p-Block Elements - 12985821 | Meritnation.com
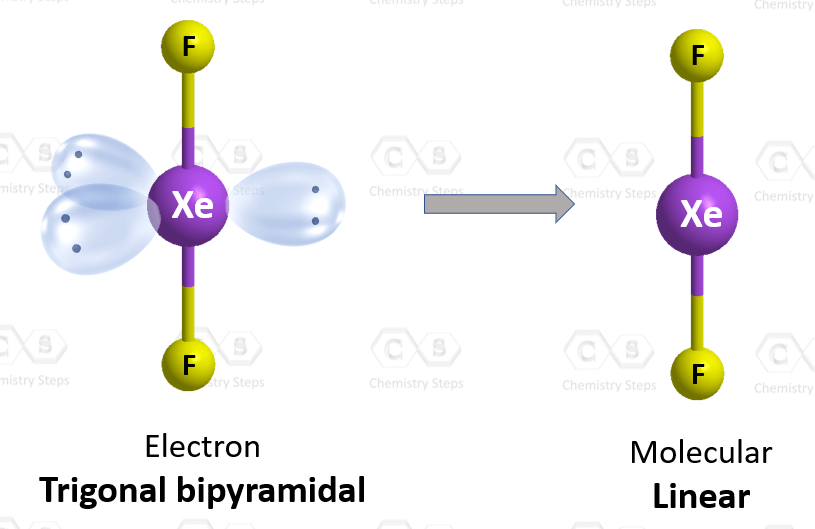
What is the shape of the XeF2 molecule, and the total number of the lone pair present on XE in a XeF2 molecule? - Quora

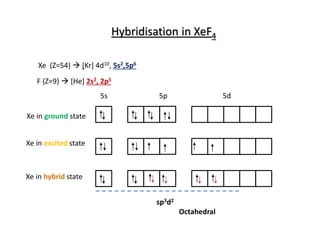
Hybridization of Xe in XeF4 is ........ and in XeF2 is ....... a. sp3, sp3d b. sp3d2, sp3d2 c. sp3d, sp3d2 d. sp3d2, sp3d e. sp3, sp3d2 | Homework.Study.com
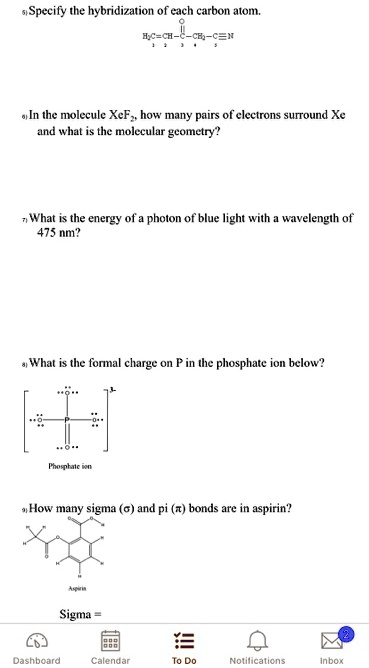
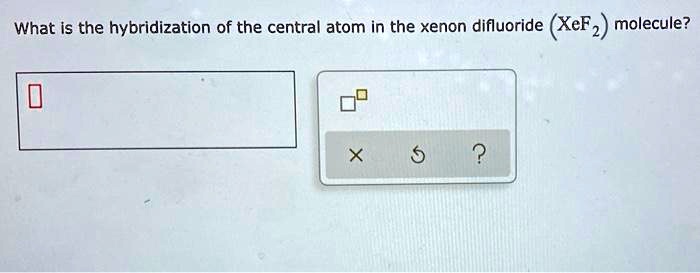
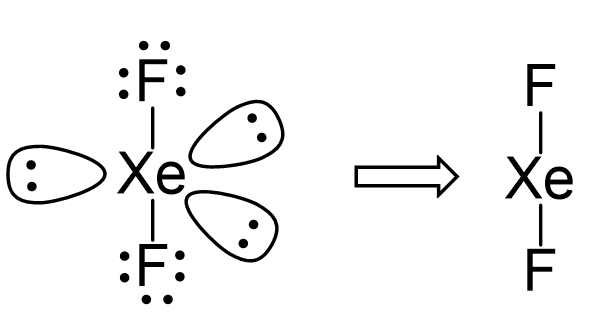
SOLVED: Specify the hybridization of the carbon atom in C=CH2. In the molecule XeF2, how many pairs of electrons surround Xe and what is the molecular geometry? What is the energy of


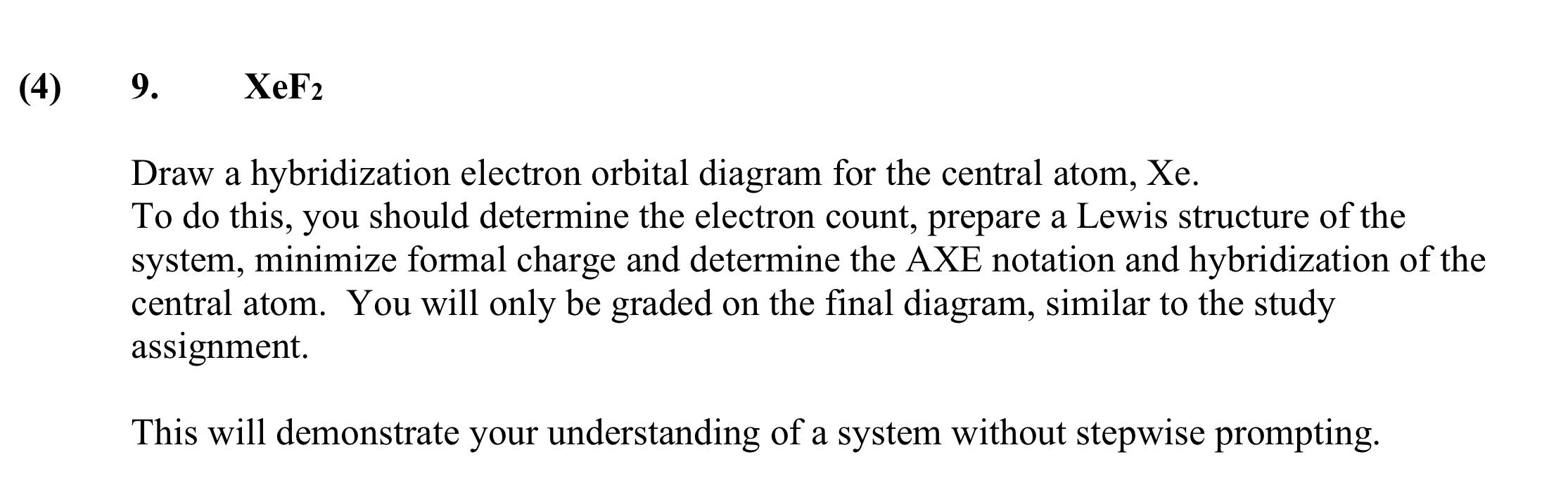
![Punjabi] Draw the structure of XeF2 Write its hybridisation? Punjabi] Draw the structure of XeF2 Write its hybridisation?](https://d10lpgp6xz60nq.cloudfront.net/physics_images/ACU_BPM_20_CHE_XII_C05_E07_003_S01.png)